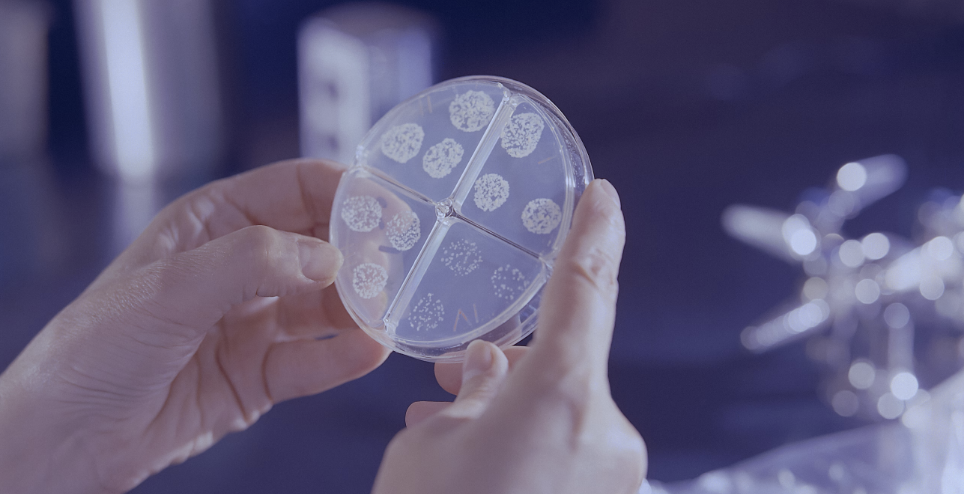
EU GMP Quality Control
European Laboratory holding MIA license and GMP certificate serving the Pharma Industry with EU Batch Release Testing, QP Batch Release, and Product Development.

Our Services
Strong Expertise in Quality Control testing of
- biological molecules
- chemical compounds
- advanced therapies
- vaccines
- drug-device combined products
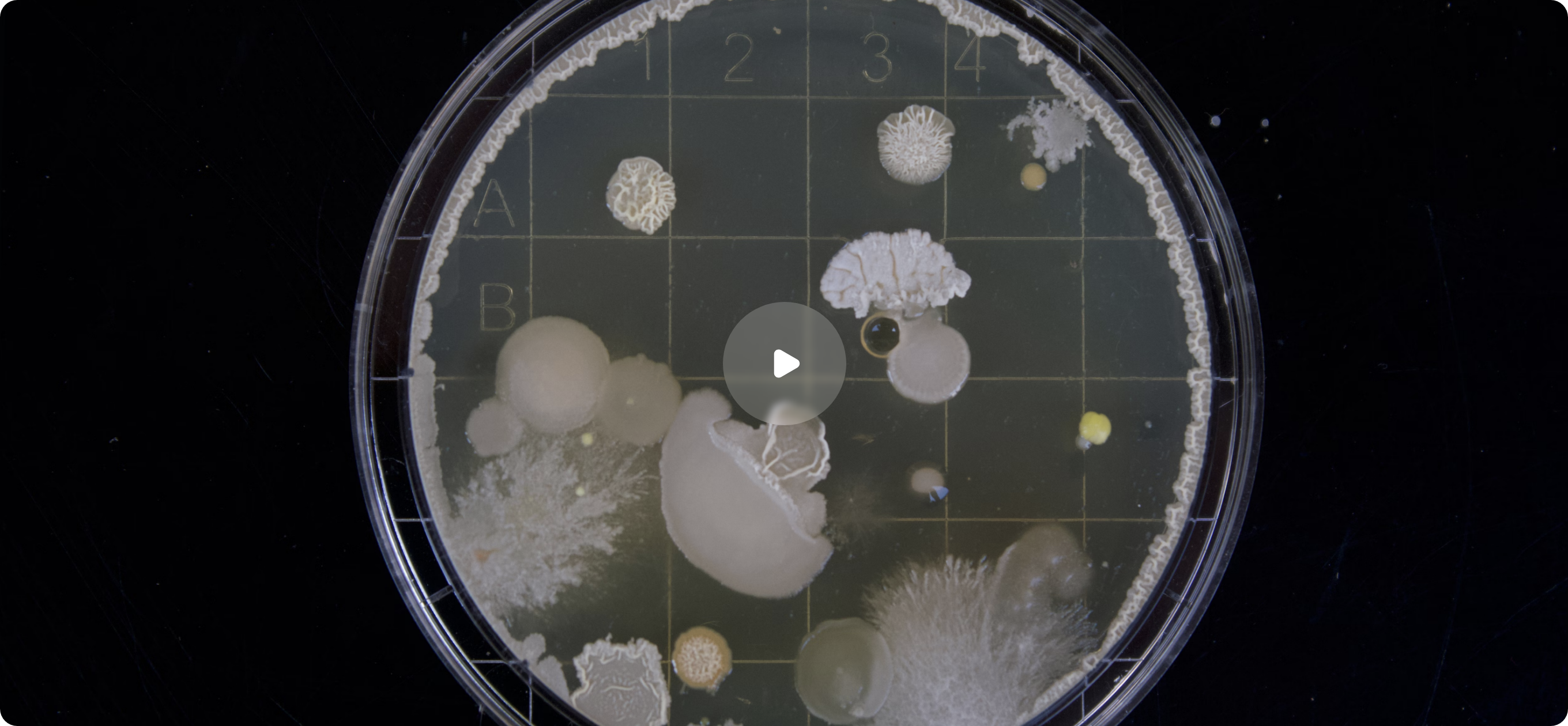
Profarma Numbers
Supporting drug product manufacturers and distributors at different stages of the drug product life cycle starting from early development, and drug registration to routine commercial supply for the EU market.
100+
Different products routinely tested
20+
Different countries
17+
Years in business
Some of Our Partners





We are always on a lookout for outstanding talent.
At Profarma, we offer various positions at our research and development, quality control, quality assurance or sales departments.
What our customers say about us
Our priority is establishing long-lasting strategic partnerships with our customer
Very good experience
“We empower our clients and aim to make the process of investing engaging and enjoyable. It’s exciting to offer the choice that modern investors demand, and the tools to keep them informed in the way they deserve. We hope they enjoy the journey as much as we do.”
Name Lastname
Co-founder and President, Company
Very good experience
“We empower our clients and aim to make the process of investing engaging and enjoyable. It’s exciting to offer the choice that modern investors demand, and the tools to keep them informed in the way they deserve. We hope they enjoy the journey as much as we do.”
Name Lastname
Co-founder and President, Company
Very good experience
“We empower our clients and aim to make the process of investing engaging and enjoyable. It’s exciting to offer the choice that modern investors demand, and the tools to keep them informed in the way they deserve. We hope they enjoy the journey as much as we do.”
Name Lastname