Profarma Named Among Gazele 2024 Fastest-Growing Companies

Profarma is proud to announce its recognition as one of the Gazele 2024 fastest-growing companies of the year, awarded by Verslo Žinios. This achievement underscores Profarma’s strong dedication to growth, innovation, and excellence in the industry. This recognition reflects the incredible contributions of the Profarma team, as well as the trust and support of our […]
Profarma Joins Lithuania Bio Association
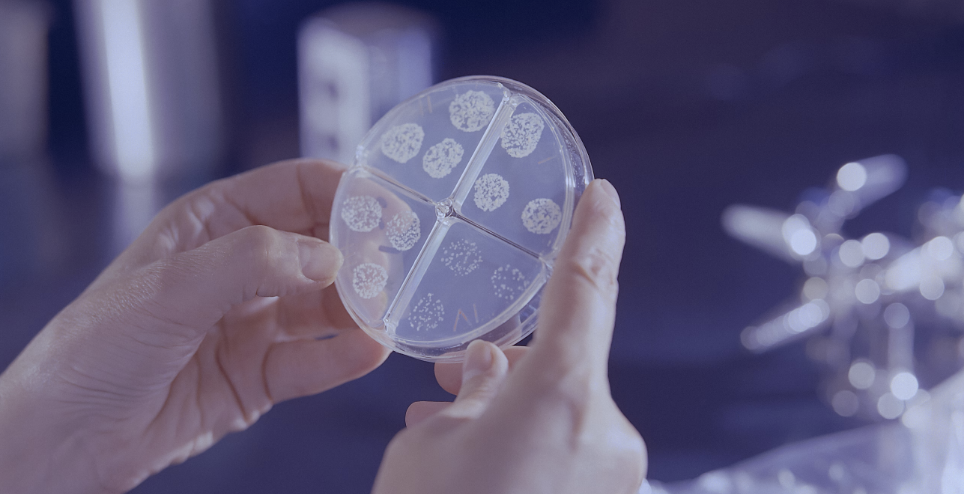
This partnership highlights Profarma’s dedication to advancing biotechnology and encouraging collaborative efforts within the industry. By joining Lithuania Bio, Profarma aims to contribute to the vibrant biotechnology community in Lithuania, leveraging the association’s platform to engage in meaningful partnerships, knowledge sharing, and joint initiatives. Lithuania Bio, renowned for its role in promoting the life sciences […]
Profarma Updates MIA License for QP Batch Release Services

Profarma is pleased to announce the successful update of its Manufacturer’s/Importer’s Authorisation (MIA) license, reinforcing our capabilities in providing Qualified Person (QP) batch release services. This update highlights our commitment in ensuring the highest levels of quality and regulatory compliance for our clients’ and tehir products. Elevated Quality Standards:With the updated MIA license, Profarma continues […]
Quality in Semaglutide and Liraglutide Batch Testing Services

Profarma is committed to providing comprehensive testing services for semaglutide and liraglutide, ensuring quality and compliance in batch release testing. Our strong understanding of EU regulations, batch release testing, and Qualified Person (QP) certification has consistently positioned us as the preferred partner for pharmaceutical companies navigating the product registration process. Quality Assurance:At Profarma, our extensive […]
Product Portfolio Expansion with New Cystic Fibrosis Treatment Project

Profarma is expanding its innovative product portfolio with the launch of a project aimed at developing advanced pharmaceutical forms and technologies for treating cystic fibrosis. Supported by funding from the European Union, this initiative marks a significant step forward in enhancing treatment options for cystic fibrosis patients worldwide. New Pharmaceutical Forms of Ivacaftor: As part […]